
Spiral wound gaskets are essentially made of two elements: a metal part generally consisting of steel or derived alloys that have the function of absorbing the loads and generating the tensional state, and a filler that allows sealing between various metal coils. |
The fillers used in spiral wound gaskets are diverse and are chosen depending on two fundamental parameters: • Operating temperature The graphite is used for its various properties given its particular crystalline structure. One of the limits of its used is its porosity, therefore not suitable for vacuum applications where PTFE is used more, thanks to its low permeability. For temperatures greater than 500°C there is a general tendency to use ceramic materials, which are usually in fibre. These materials are used only for very high temperatures.
Graphite PTFE Polytetrafluoroethylene is a polymer with high specific weight characterized by the bond Fluoro Carbon. This type of bond is particularly stable, because fluorine, being at the extreme left of periodic table, has a high electro-negativity and therefore the electronic cloud is very compact and predominantly placed on the fluorine atom. For these characteristics of high stability bond and compactness, the resulting polymer shows a number of properties that can we summarize below: • High thermal stability compared to other polymers on the market. • Resistance to chemical solvents • Very low gas permeability • Low friction coefficient • High anti-adhesiveness. • Excellent dielectric resistance It takes advantage of its high chemical inertia. The use of PTFE is compatible with the majority of known chemicals except with the salts of alkali cast metals and precursors of fluorine. The use temperature can reach up to 260° C, very high compared to common polymers. Given its low permeability it is used as a filler for gaskets used in vacuum services. Chlorite Minerals Ceramic Fibres Formed by aluminium silicate fibres. These materials have a low sealant effect compared to other fillers, and therefore have an excellent temperature stability up to 2300° F (1250° C). They are resistant to attack by many corrosive agents (hydrofluoric acid and phosphoric acid), including alkali. They are recommended when you can not use graphite filler or PTFE.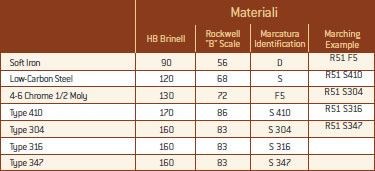
|

